ENM Characterisation
 ENM characterisation with the use of advanced microscopy and spectroscopy techniques underpins all the experimental work in NanoFASE. Pristine particles are characterised prior to experiments. This includes particles supplied from industry or specially prepared for NanoFASE experiments including artificially aged particles. Where possible particle transformations are studied in environmental media in real time (for example agglomeration or aggregation), or after experiments (for example in bio-uptake experiments). This adds realism to the conclusions drawn about nanomaterials fate and exposure in the environment, an important contribution of NanoFASE which builds on the many earlier fate studies involving pristine particles in less realistic media.
ENM characterisation with the use of advanced microscopy and spectroscopy techniques underpins all the experimental work in NanoFASE. Pristine particles are characterised prior to experiments. This includes particles supplied from industry or specially prepared for NanoFASE experiments including artificially aged particles. Where possible particle transformations are studied in environmental media in real time (for example agglomeration or aggregation), or after experiments (for example in bio-uptake experiments). This adds realism to the conclusions drawn about nanomaterials fate and exposure in the environment, an important contribution of NanoFASE which builds on the many earlier fate studies involving pristine particles in less realistic media.
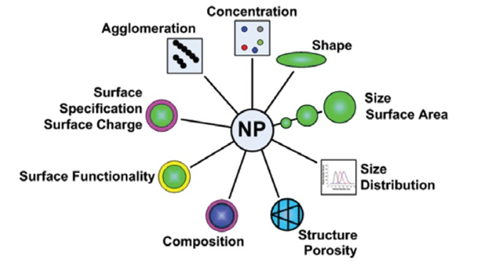
Figure 1. Important characteristics of nanoparticles.
Data is collated on the NanoFASE-tailored Biomax portal. In addition to the physical and chemical properties of particles, characterisation of ENM ecological/biological properties such as acquired biomolecule corona, interactions with environmental molecules, attachment to soil / heteroaggregation, bioaccumulation and mesocosms are found in the portal. Methods used, developed and optimised (including improvements to sampling, pre-treatment and analytics) in NanoFASE to allow ENM characterisation in complex environmental media include:
Light scattering methods (used for measuring particle size and size distribution)
Nanoparticle tracking Analysis (NTA)
NTA is used to obtain the nanoparticle size distribution of samples in liquid suspension. The advantage of this method over other light-scattering methods is that many particles are individually tracked (particle-by-particle) enabling count-based concentration and aggregation measurements in real time. In NanoFASE NTA has been utilised to characterise and quantify the presence of the EMNs in complex environmental samples such as groundwater. Malvern Panalytical, a partner in NanoFASE, developed an AutoSampler for rapid, automated analysis.
Dynamic Light Scattering (DLS)
DLS is used to obtain nanoparticle size distribution of samples in liquid suspension. The advantage over other light-scattering methods is the minimum measureable particle size < 10 nm. DLS is widely used in NanoFASE to characterise pristine nanoparticles. The ability to measure Zeta potential is often combined with DLS instruments and used to measure colloidal stability.
Differential Centrifugal Sedimentation (DCS)
DCS is used to obtain nanoparticle size distribution of samples in liquid suspension. The advantage over other light-scattering methods is the ability to resolve multi modal particle distributions via the application of a centrifugal force. The method is also known as Centrifugal Separation Analysis (CSA) and is being used by NanoFASe partners UniVIE to investigate the environmental behaviour, especially transformation processes, of CuO nanoparticles in fresh waters.
Mass Spectrometry (used to determine elemental composition and concentration)
Inductively coupled plasma mass spectrometry (ICP-MS)
ICP-MS is used to accurately detect and obtain the concentration of trace metals in a liquid (dissolved) sample. It is widely used in NanoFASE where applications include determining the amount of silver released from textiles in washing as well as in bioaccumulation studies. The disadvantage of the method is that the sample is destroyed during the analysis.
Single particle ICP-MS (sp-ICP-MS)
Single-particle ICP-MS is used to detect, characterise and quantify nanoparticles in liquid suspensions. spICP-MS can distinguish between ionic and particulate forms of an element in the same sample. It is used in NanoFASE to quantify nano particulate forms versus dissolved material in environmental samples (for example the case study on biokinetics of AG-NPs in isopods), or to distinguish nanoforms from ions in the copper nanowires pesticide case study.
Microscopy (used to identify ENM in samples and determine particle size, size distribution, shape and morphology)
Scanning Electron Microscopy (SEM)
SEM produces images of a sample by scanning the surface with a focused beam of electrons. The electrons interact with atoms in the sample, producing various signals that contain information about the surface topography and composition of the sample. Elemental contrast can be obtained by using a backscattered imaging mode. Environmental SEM (ESEM) is used to image samples in a more natural state (undried and uncoated). State of the art SEMs have a spatial resolution better than 1 nm. SEM and TEM (see below) have been used in NanoFASE to study ENM release for example from household washing of nanoenabled textiles.
Transmission Electron microscopy (TEM)
TEM produces images from a beam of electrons transmitted through an ultrathin sample or a suspension precipitated onto a grid. TEM is used to determine nanoparticle size, number, shape and morphology in both pristine samples and environmental samples. Care with biological sample preparation is important. Scanning transmission electron microscopy (STEM) uses a fine electron probe in scanning transmission mode to obtain images with a good Z (elemental) contrast and can be combined with EDX for additional chemical information. High Resolution TEM (HRTEM) allows for direct imaging of the atomic structure of a material (spatial resolution > 0.050 nm). It was used in NanoFASE case studies including fate assessment of nano copper hydroxide nanowires in a pesticide application.
TEM and SEM have been used in NanoFASE for the characterisation of most starting materials and some products of environmental modification in air, water, soil and within the mesocosm experiment.
Spectroscopy
Ultraviolet-visible (UV-Vis) Spectroscopy
Measurement of absorption of radiation in the UV or visible range can be used to measure the presence of certain chemicals generally in liquid. In NanoFASE it is used to determine the size and concentration of gold nanoparticles in environmental media.
Raman Spectroscopy
Raman spectroscopy is used for compositional and structural analysis. The advantage of this optical method, often combined with microscopy, is that analysis can be done on environmental samples without further preparation. This was used in NanoFASE to determine the chemical phase of TiO2 on a pebble sample recovered from a river following a paint spill.
Fourier Transform Infrared (FTIR) Spectroscopy
FTIR is used to determine molecular structure, in particular for the identification of organic species. The Attenuated Total Reflection (ATR) variant enables samples to be examined directly in solid or liquid state without further sample preparation. Diffuse Reflectance Infrared Fourier Transform Spectroscopy (DRIFTS) is another FTIR sampling method which can be used on powder samples. Mid Infrared (MIR) spectroscopy can be used as a tool for soil classification by identification of inorganic components such as carbonates.
Energy Dispersive X-ray spectroscopy (EDX)
EDX is used for chemical characterization of a sample (elemental analysis) and is used with SEM and TEM. Electron excitation leads to emission of characteristic X-rays unique to each element. EDX mapping can be performed showing the distribution of an element in a sample. EDX on a state of the art TEM is capable of single atomic resolution concurrent with imaging. In NanoFASE most of the SEM and TEM studies are combined with EDX.
X-ray Absorption Spectroscopy (XAS)
XAS is used to determine the chemistry and local structure of an element in a sample. Extended X-Ray Absorption Fine Structure (EXAFS) provides information on bond distances and co-ordination to neighbouring atoms. X-ray Absorption Near Edge Structure (XANES) provides information on the chemical state of the element. Because a tunable, high flux X-ray source is required, these techniques require the use of a synchrotron. NanoFASE gained access to synchrotrons in two hemispheres to investigate how soil properties can influence the uptake (in wheat) of silver (Ag) from different Ag forms. XAS been also used in NanoFASE to investigate the transformation of Ceria ENMs in sewage sludge incineration for example.
X-ray photoelectron spectroscopy (XPS)
XPS is used to determine the chemical composition from the surface of a sample (5-10 nm). The sample is irradiated with soft X-rays (energy ~ 1500 eV) exciting core electrons which have energy sufficient only to escape from the near surface. The concentration of each element (except hydrogen and helium) is determined from the intensity of the photoelectron peak and the chemistry is determined from shifts in the peak position. In addition XPS can be used to determine surface functionality of nanoparticles and thickness of surface layers.
Diffraction (used to determine chemical composition and crystallographic structure)
X-ray Diffraction (XRD)
Read more |
Read also |
|
http://nanofase.eu/news/1174_nanofase-uses-the-biomax-platform-for-project-traceability/
|
Analysis of SiO2 Nanoparticles in Standard Mode with Single Particle ICP-MS. PerkinElmer Application Note, undated. Overview of chemical imaging methods to address biological questions https://doi.org/10.1016/j.micron.2016.02.005 Myhra S & Riviere J (2013) Characterisation of NanoStructures. Boca Raton, FL: CRC Press. ISBN 9781138198630 |
Contact
 Alison Crossley
Alison Crossley
Oxford Materials Characterisation Service
Oxford University
Email: alison.crossley@materials.ox.ac.uk
 Kerstin Jurkschat
Kerstin Jurkschat
Oxford Materials Characterisation Service
Oxford University
