Sulfidation rate constant
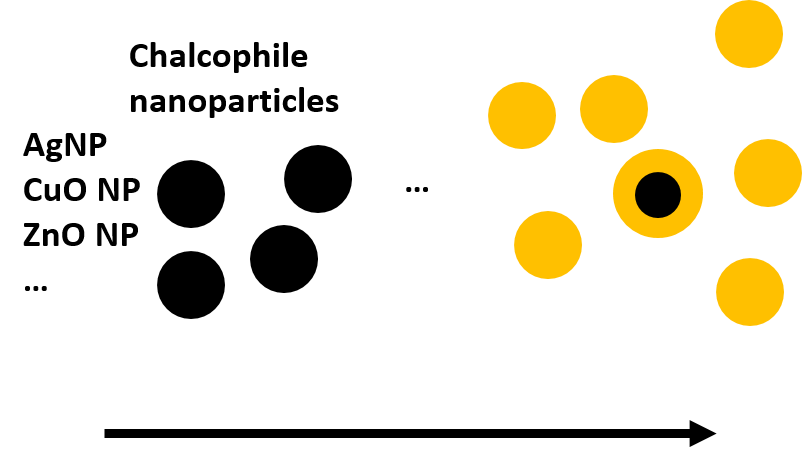
Describes the rate at which a metal-based nanoparticle reacts with available sulfide to form metal sulfides.
|
|
Used for |
|
Algorithms |
Procedure |
|
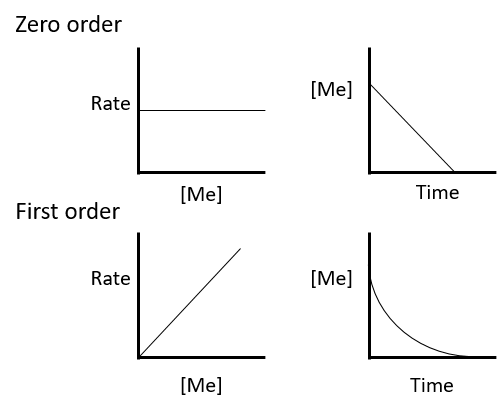
Where \(F_{CuO,t}\) is the remaining fraction of \(CuO\) at time \(t\) of the reaction of \(CuO\) with \(HS^{-}\), \(k\) is the Pseudo first-order reaction rate coefficient and \(t\) is the time. Pseudo first-order reaction rate coefficient \(k\) is defined as: |
Colorimetric method using Zincon X-ray absorption spectroscopy |
Read more |
Read also |
|
A. Gogos, B. Thalmann, A. Voegelin and R. Kaegi, Sulfidation kinetics of copper oxide nanoparticles, Environmental Science: Nano, 2017, 4, 1733-1741. |
B. Thalmann, A. Voegelin, E. Morgenroth and R. Kaegi, Effect of humic acid on the kinetics of silver nanoparticle sulfidation, Environmental Science: Nano, 2016, 3, 203-212. R. Kaegi, A. Voegelin, C. Ort, B. Sinnet, B. Thalmann, J. Krismer, H. Hagendorfer, M. Elumelu and E. Mueller, Fate and transformation of silver nanoparticles in urban wastewater systems, Water research, 2013, 47, 3866-3877. |
Contact
 Alexander Gogos
Alexander Gogos
EAWAG, Switzerland