X-ray absorption spectroscopy (XAS)
X-ray Absorption (fine structure) spectroscopy enables studying, at the atomic and molecular scale, the local structure around selected elements contained within a material. The basic physical quantity that is measured in XAS is the X-ray absorption coefficient μ(E), which describes how strongly X-rays are absorbed as a function of X-ray energy E.
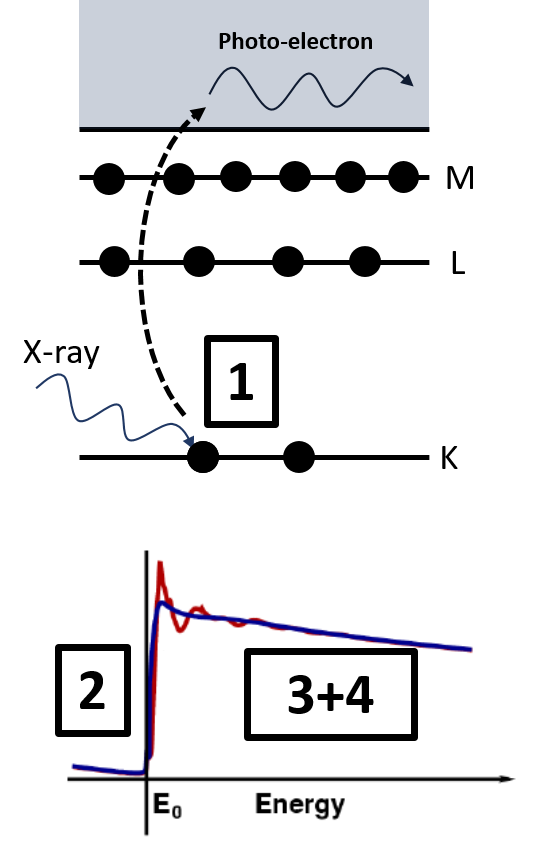 1. A core-level electron is excited by high energy X-rays and promoted to the continuum as a photo-electron. The energy that is needed to eject this core-electron by absorption of the x-ray is element specific and discrete.
1. A core-level electron is excited by high energy X-rays and promoted to the continuum as a photo-electron. The energy that is needed to eject this core-electron by absorption of the x-ray is element specific and discrete.
2. At the element specific E0 (the absorption edge), a sharp rise in the absorption coefficient will occur.
3. As there is never a free standing atom (blue line), the photo-electron will be scattered back by the other atoms that surround the atom.
4. This will then again modulate the absorption coefficient, resulting in oscillations (red line). These contain the information on the chemical environment.
Read also |
|
Bunker, G. (2010). Introduction to XAFS: a practical guide to X-ray absorption fine structure spectroscopy. Cambridge University Press. |
Contact
 Alexander Gogos
Alexander Gogos
EAWAG, Switzerland
