NanoFASE Pristine Particle: Dysprosium-labelled Copper Hydroxide Nanowires
Copper hydroxide [Cu(OH)2] nanoforms are commercially available as a pesticide for agricultural use.
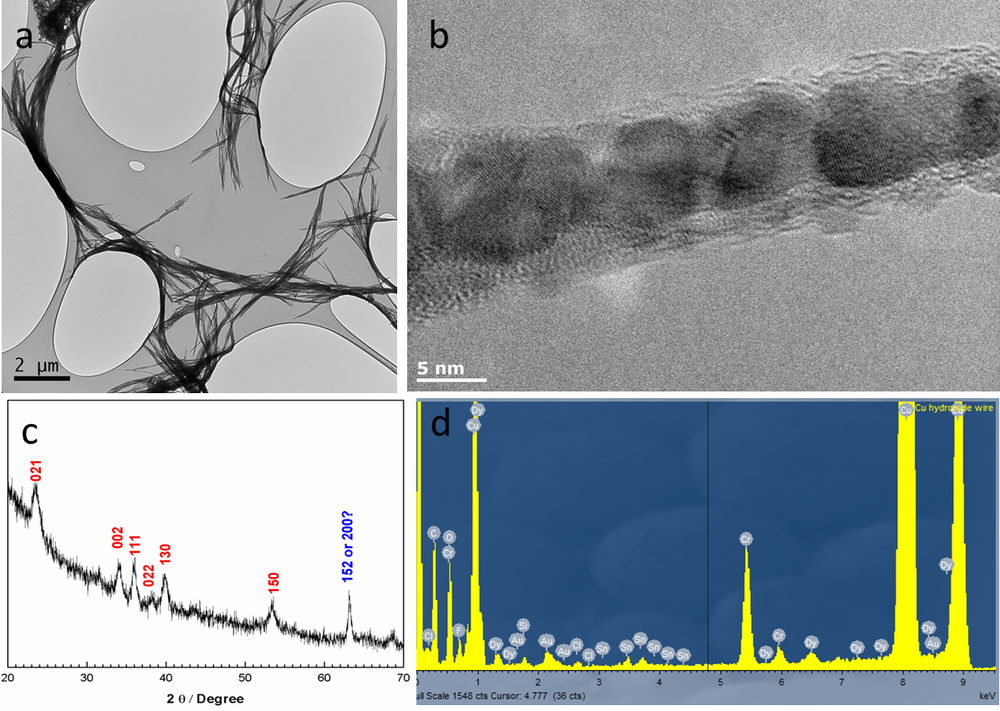
NanoFASE looked at the fate of copper-based nanowires as a pesticide application. For the requirements of this experiment, partner University of Birmingham produced Dysprosium-doped copper hydroxide Nanowires nanowires [Dy-Cu(OH)2 NWs] with a polyethylene glycol (PEG) coating to stabilise. These are designed to mimic commercial Cu(OH)2 pesticides but also enable tracking at low concentrations and allow assessment of bio-uptake form (NW versus Cu2+ ions). Mean particle diameter was ~ 20nm and mean length was > 10 microns.
All Dy signal detected was associated with Cu(OH)2 nanowires, but the Dy was not homogeneously distributed.
Synthesis procedure
8 mmol Cu(NO3)2 and 0.2 mmol DyCl3•6H2O were dissolved in 100 mL H2O in the presence of 4 g m-PEG (MW 5000) under stirring at room temperature to form a clear light blue solution. To the Cu/Dy solution, 20 mL 10 mol/L NaOH solution was quickly added. The blue slurry was left under stirring at room temperature for over 4 hours. The blue precipitate was collected by centrifuge, and washed with H2O three times.
|
|
Occurs in |
|
|
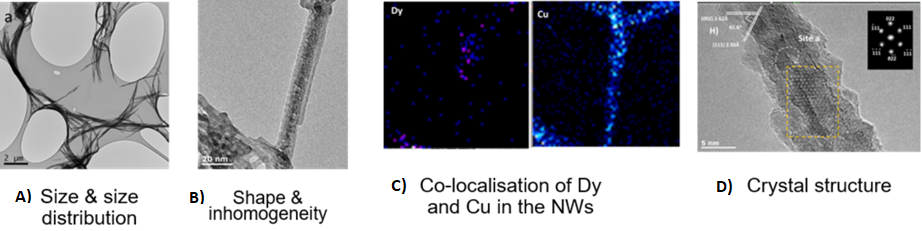
Figure 2. A) TEM (supported by spICP-MS analysis and conversion of mass to volume to size; see also spICP-MS procedure; B) TEM; C) Confocal microscopy and/or EDX mapping in TEM, and following digestion of tissue, by detection of both elements using ICP-MS; D) High resolution TEM.
Read more |
Read also |
|
NanoFASE Case Study: Fate of Cu(OH)2 nanowires utilised as pesticides Unique NanoFASE Knowledge Base ID number=NP00686 |
Kent RD & Vikesland PJ (2016) Dissolution and Persistence of Copper-Based Nanomaterials in Undersaturated Solutions with Respect to Cupric Solid Phases. Environ. Sci. Technol. 2016, 50, 13, pp. 6772-6781. https://pubs.acs.org/doi/abs/10.1021/acs.est.5b04719 |
Contact
 Xianjin Cui
Xianjin Cui
 Iseult Lynch
Iseult Lynch
Email: i.lynch@bham.ac.uk