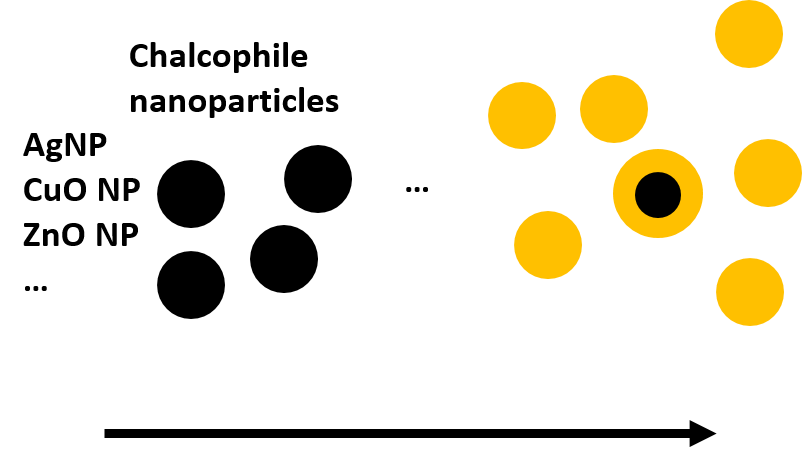
Sulfidation
The process of sulfidation describes the chemical reaction of a chalcophile metal with bisulfide (HS-) forming a metal sulfide as the final reaction product. Bisulfide is present in anoxic aqueous environments where it is formed through sulfate reduction during decomposition of organic matter. Such environments include for example waste water systems (sewage systems and treatment plants WWTPs) and sediments. Bisulfide concentrations can be particularly high – from a few µg L-1 up to tens of mg L-1 – in sewage systems and WWTPs, to which a considerable fraction of ENPs will be released.
Wastewater systems including sewage system and WWTP
Fate descriptors |
Algorithms |
|
\(CuO\)
|
Pseudo first-order model The sulfidation rate for CuO NP is described by the following equation: \(F_{CuO,t}=e^{-k}\) Where \(F_{CuO,t}\) is the remaining fraction of \(CuO\) at time \(t\) of the reaction of \(CuO\) with \(HS^{-}\), \(k\) is the Pseudo first-order reaction rate coefficient and \(t\) is the time. Parabolic rate model The sulfidation rate for Ag NP reacted with HS in the presence of humic acids is described by the following equation where r is the nanoparticle radius: \(F_{Ag,t}=(1-\sqrt\frac{k}{r^{2}}.t)^{3}\) |
Read more |
Read also |
|
Gogos, A., B. Thalmann, A. Voegelin and R. Kaegi (2017). "Sulfidation kinetics of copper oxide nanoparticles." Environmental Science: Nano 4(8): 1733-1741. B. Thalman, A. Voegelin, E. Morgenroth and R.Kaegi, Effect of humic acid on the kinetics of silver nanoparticle sulfidation, Environmental Science: Nano, 2016, 3, 2013-212. |
|
Contact
 Alexander Gogos
Alexander Gogos
EAWAG, Switzerland
