Nanomaterials transformations in the environment
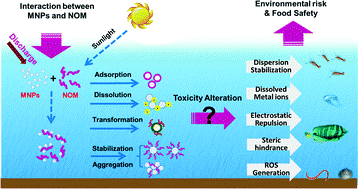
The potential transformations undergone by ENMs in the environment have been grouped into 4 main categories, namely physical transformations, chemical transformations, biological transformations, and corona formation. More than one of these transformations can occur simultaneously and it is not always clear which dominates under specific environmental conditions.
In terms of the Functional Fate Group concept utilised in NanoFASE as a means to simply the diversity of ENMs, we have focussed on two main types of transformations. The first is chemical transformation due to reactions at the ENM surface, such as sulfidation or phosphatation. The second is corona formation as a result of the acquisition of natural organic matter or other small or large biomolecules onto the surface of the ENMs, which confers an ecological identity to the ENMs allowing them to interact specifically with biological receptors and ecological organisms.
Chemical transformations |
|
SulfidationSulfidation is a chemical reaction of metal or oxide with sulfur in some form in the environment. While it presents a problem in corrosion of engineering components, in the case of ENMs sulfidation has been demonstrated to be a means of reducing the ENM toxicity by preventing the release of toxic ions. Silver ENMs readily react with sulfide to form Ag(0)/Ag2S core–shell particles: due to the lower solubility of Ag2S relative to elemental Ag (Ag0) even partially sulfidized Ag ENMs have lower toxicity than pristine Ag ENMs (Levard et al, 2016). PhosphatationThe influence of phosphate on the dissolution and microstructural transformation of ZnO ENMs was investigated in the NanoMILE project: Phosphate at a low concentration rapidly and substantially reduced the release of Zn2+ into aqueous solution. Interaction between ZnO ENMs and phosphate induced the transformation of ZnO into zinc phosphate. TEM observation shows that the morphology of the particles changed from structurally uniform nanosized spherical to anomalous and porous material containing mixed amorphous and crystalline phases of ZnO and zinc phosphate in the presence of phosphate. Similar effects have been found with Cerium dioxide (CeO2) EMNs and with a series of increasingly Zr-doped CeO2 ENMs where the spherical particles were rapidly transformed into sea urchin-like structures up to a micron in size, with the Zr doping having no effect on this transformation of CeO2 into CePO4 (Briffa et al., 2019). OxidationFor ENMs, and in NanoFASE, oxidation behaviour of zero valent iron EMNs (nZVI) has been investigated. Using HR-TEM Kumar et al. (2014) showed that a surface oxide layer (∼3 nm) formed immediately after the nZVI particles were exposed to water. The metallic iron concentration in nZVI particles exposed to water was ∼40% after 35 days. The results suggested that nZVI oxidized via the Fe(0) - Fe(OH)2 - Fe3O4 - (γ-Fe2O3) route and the formation of superparamagnetic maghemite ENMs due to disruption of the surface oxide layer. DissolutionChloride is well known to facilitate the dissolution of, for example, Ag ENMs, and as such standard high salt media often need to be diluted to prevent rapid dissolution of the Ag ENMs. Chloride (Cl–) induced dissolution is challenging to study because of the numerous soluble and solid Ag–Cl species that can form depending on the Cl/Ag ratio. The kinetics of dissolution are strongly dependent on the Cl/Ag ratio and can be interpreted using the thermodynamically expected speciation of Ag in the presence of chloride. |
|
Corona formation |
|
|
The term corona describes the layer of biomolecules that adsorbs to ENM surfaces in contact with the environmental compartments (air, water, sediment, soil, WWTPs). The biomolecules available to bind to ENMs include natural organic matter (NOM), which is the breakdown products of plants and animals in the environment, as well as biomolecules secreted by organisms as part of their normal signalling and digestive processes. While the role of NOM in stabilising ENMs is well established, the role of the adsorbed biomolecule corona in determining ENM fate and toxicity is less well understood, and an area of intense investigation.
Figure: Wang et al. (2006) https://doi.org/10.1039/C5EN00230C The nature of the biomolecules that will potentially bind to specific ENMs is related to the range of available large and small biomolecules as well as to the physico-chemical characteristics of the ENM (Lynch et al., 2014). A core aspect of NanoFASE has been the development of protocols for studying eco-coronas in WWTP fluids, as well as characterisation of ENM coronas in sludge, soil pore water, aquatic media and following elimination of ENMs from organisms. These latter experiments were performed within NanoFASE to parameterize the Water-Soil-Organism model. |
Read more |
Read also |
|
Visit the NanoFASE Library: NanoFASE Reports D3.5 and D3.6 (awaited) Gogos A, Thalmann B, Voegelin A, Kaegi R. Sulfidation kinetics of copper oxide nanoparticles. Environ. Sci.: Nano, 2017, 4, 1733-1741. Gogos A, Voegelin A, Kaegi R. Influence of organic compounds on the sulfidation of copper oxide nanoparticles. Environ. Sci.: Nano, 2018, 5, 2560-2569. |
Briffa SM, Lynch I, Hapiuk D, Valsami-Jones E. Physical and chemical transformations of zirconium doped ceria nanoparticles in the presence of phosphate: Increasing realism in environmental fate and behaviour experiments. Environ. Poll., 2019, Sep; 252(Pt B):974-981. DOI: 10.1016/j.envpol.2019.06.014 Briffa SM, Lynch I, Trouillet V, Bruns M, Hapiuk D, Valsami-Jones É. Thermal Transformations of Manufactured Nanomaterials as a Proposed Proxy for Ageing. Environ. Sci.: Nano, 2018, 5, 1745-1756. Briffa SM, Nasser F, Valsami-Jones E, Lynch I. Uptake and impacts of polyvinylpyrrolidone (PVP) capped metal oxide nanoparticles on Daphnia magna: role of core composition and acquired corona. Environ. Sci.: Nano, 2018, 5, 1745-1756. Kumar N, Auffan M, Gattacceca J, Rose J, Olivi L, Borschneck D, Kvapil P, Jublot M, Kaifas D, Malleret L, Doumenq P, Bottero JY. Molecular insights of oxidation process of iron nanoparticles: spectroscopic, magnetic, and microscopic evidence. Environ Sci Technol. 2014, 48: 13888-94. Levard C, Mitra S, Yang T, Jew AD, Badireddy AR, Lowry GV, Brown GE Jr. Effect of Chloride on the Dissolution Rate of Silver Nanoparticles and Toxicity to E. coli. Environ. Sci. Technol., 2013, 47 (11), pp 5738–5745. Lowry GV, Gregory KB, Apte SC, Lead JR. Transformations of Nanomaterials in the Environment. Environ. Sci. Technol. 2012, 46: 6893-6899. Lv J, Zhang S, Luo L, Han W, Zhang J, Yang K, Christie P. Dissolution and Microstructural Transformation of ZnO Nanoparticles under the Influence of Phosphate. Environ. Sci. Technol., 2012, 46, 7215–7221. Lynch I, Valsami-Jones E, Lead JR, Dawson K. Macromolecular coronas and their importance in nanotoxicology and nanoecotoxicology. Nanoscience and the Environment, 1st Edition, 2014, 7: 127-156. Frontiers of Nanoscience. Editors: Lead JR & Valsami-Jones E. Markiewicz M Kumirsk J, Lynch I, Matzke M, Köser J, Bemowsky S, Docter D, Stauber R, Westmeier D, Stolte S. Changing environments and biomolecule coronas: Consequences and challenges for the design of environmentally acceptable engineered nanoparticles. Green Chemistry, 2018, 20, 4133-4168. Nasser F, Lynch I. Secreted protein eco-corona mediates uptake and impacts of polystyrene nanoparticles on Daphnia magna. J Proteomics, 2016, 137, 45-51. Tejamaya M, Römer I, Merrifield RC, Lead JR. Stability of Citrate, PVP, and PEG Coated Silver Nanoparticles in Ecotoxicology Media. Environ. Sci. Technol., 2012, 46, 7011–7017. Zhang F, Allen AJ, Johnston-Peck AC, Liu J, Pettibone JM.*Transformation of engineered nanomaterials through the prism of silver sulfidation. Nanoscale Adv., 2019, 1, 241-253. |
Contact
 Iseult Lynch
Iseult Lynch
Email: i.lynch@bham.ac.uk