Redox transformations
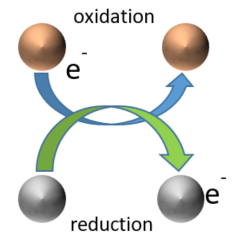
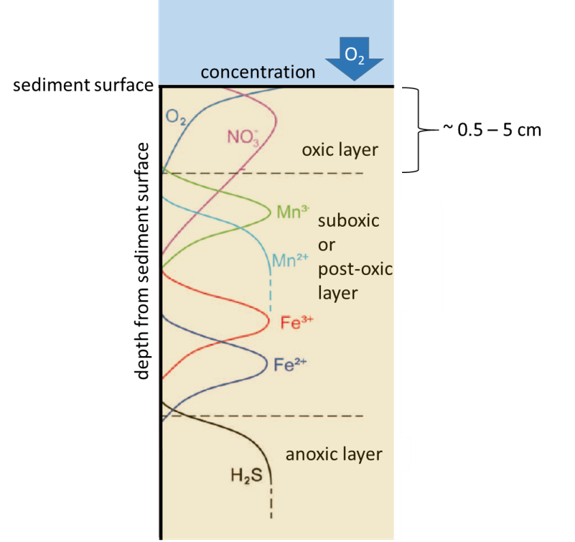
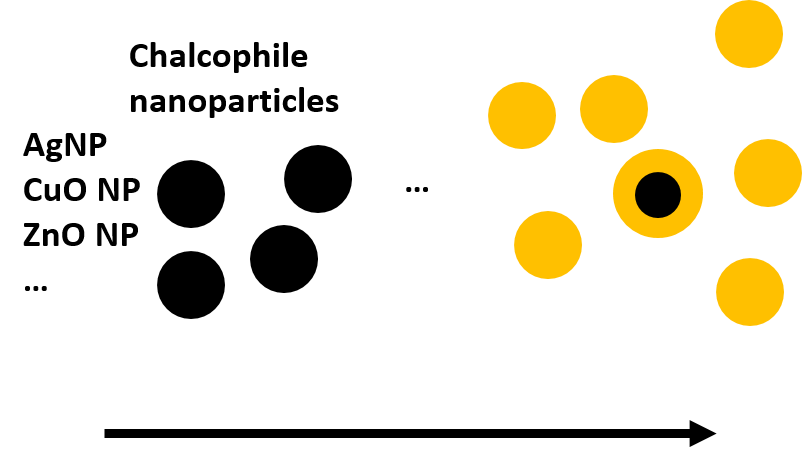
Oxidation-reduction processes, corresponding to the transfer of electrons between chemical species, play a major role in the biogeochemical cycle of trace elements and contaminants. In sediments, soils and surface waters, the presence of organic carbon is favorable to the development of microorganisms, coupling the mineralization of organic carbon (i.e. the oxidation process) with reduction processes involving different electron acceptors, such as oxygen. In anoxic environments with limited oxygen supply, such as sediments and water-saturated soils, microorganisms use other electron acceptors such as nitrate, iron, manganese and sulfates, which is called anaerobic respiration. In principle all these reactions transfer electrons from the organic carbon (which is oxidized in this process) to other substances such as oxygen or nitrate (which are reduced in this process). Since these reactions are always coupled, they are called redox reactions from reducing-oxidizing. Among these redox reactions, the reduction of sulfate SO42- to sulfide (HS-,H2S, S2-) can have a strong impact on the fate of some nanoparticles, such as silver and copper, that can be rapidly transformed into silver- and copper sulfide particles, which are reportedly less reactive than the original particles.
|
|
Occurs in |
|
|
|
|
Fate descriptors |
Algorithms |
|
|
|
|
Read more |
|
|
Consult the NanoFASE Library to see abstracts of these deliverable reports: |
Contact
 Frank von der Kammer
Frank von der Kammer
University of Vienna, Austria
Email: frank.von.der.kammer@univie.ac.at
 Lucle Stetten
Lucle Stetten
University of Vienna, Austria