Diffusion
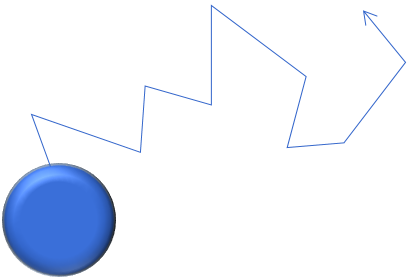
Diffusion of particles refers to their movement in liquid or gas. Particles suspended in a liquid or a gas are in continuous interaction with the surrounding liquid or gas molecules. The latter are in constant random motion (Brownian motion), meaning that small suspended particles (especially those of less than approximately 100 nm) are consequently also in constant random zigzag motion, leading to homogenous diffusion as well as increased frequency of particle coagulation and attachment (see also atmospheric dry deposition).
|
|
Occurs in |
||
|
|
|
 Water |
|
Fate descriptors |
Algorithms |
|
|
Root mean square displacement |
| Particle size Temperature |
Read more |
Read also |
|
|
W. Hinds (1999), Aerosol Technology – Properties, Behavior, and Measurement of Airborne Particles, John Wiley & Sons.
A. Einstein (1905), Über die von der molekularkinetischen Theorie der Wärme geforderte Bewegung von in ruhenden Flüssigkeiten suspendierten Teilchen, Ann. Phys. 17: 549-560.
|
Contact

Christof Asbach
Institut für Energie- und Umwelttechnik (IUTA)
Email: asbach@iuta.de