Coagulation
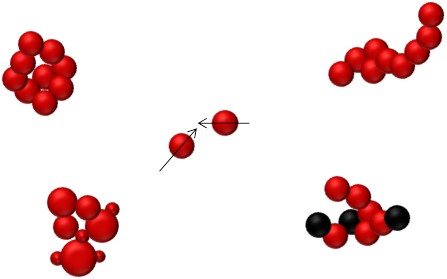
Coagulation is understood as a process originating from the collision of particles. When two particles collide, van der Waals forces cause them to stick together to form one larger agglomerate. As a result the number concentration of airborne particles decreases whereas the mean particle size increases. The collision frequency between particles is a function of the particle concentration and particle size, besides thermodynamic conditions of the gas.
An increased particle concentration increases the likelihood of particle collisions. Given that collisions only occur in case of particles moving relative to each other, small particles are much more prone to coagulation because of their constant random Brownian motion.
|
|
Occurs in |
|
|
Fate descriptors |
Algorithms |
|
Particle size |
Particle concentration |
|
|
Read more |
Read also |
|
NanoFASE Report D2.1 Specification for the NanoFASE model |
W. Hinds (1999), Aerosol Technology – Properties, Behavior, and Measurement of Airborne Particles, John Wiley & Sons. |
Contact

Christof Asbach
Institut für Energie- und Umwelttechnik (IUTA)
Email: asbach@iuta.de