Zeta potential
Zeta potential is a measure of the magnitude of the electrostatic or charge repulsion/attraction between particles, and is one of the fundamental parameters known to affect colloidal stability. It is typically measured using Electrophoretic Light Scattering (ELS).
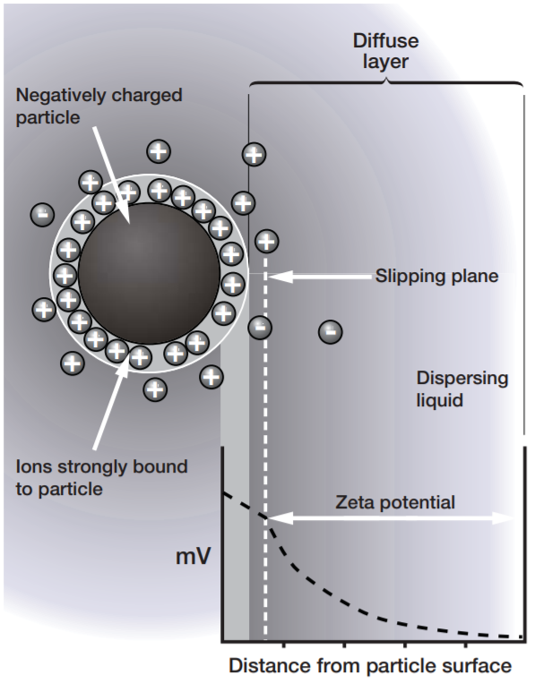 |
When a charged particle is suspended in a liquid, ions of opposite charge will be attracted to the surface of the suspended particle. A negatively charged sample attracts positive ions from the liquid and conversely a positively charged sample attracts negative ions from the liquid. Ions close to the surface of the particle will be strongly bound while ions further away will be loosely bound forming a ‘diffuse’ layer. Within the diffuse layer there is a notional boundary and any ions within this boundary will move with the particle when it moves in the liquid; but any ions outside the boundary will stay where they are – this boundary is called the slipping plane. A potential exists between the particle surface and the dispersing liquid which varies according to the distance from the particle surface – this potential at the slipping plane is called the zeta potential. The magnitude of the zeta potential gives an indication of the potential stability of the colloidal system. |
Read more |
Read also |
|
https://www.malvern.com/en/products/measurement-type/zeta-potential |
ASTM E2865 (2012) Standard Guide for Measurement of Electrophoretic Mobility and Zeta Potential of Nanosized Biological Materials. ASTM International, West Conshohocken, PA. www.astm.org
|
Contact

Phil Vincent
Malvern Panalytical
Email: Phil.vincent@malvern.com
