Particle diffusivity in air
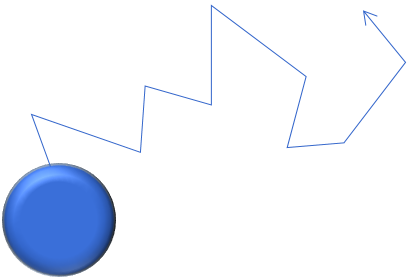
Airborne particles are in constant random Brownian motion, due to interaction with the surrounding gas molecules. Upon collision, a molecule transfers momentum to the particle, causing the particle to move. Due to the random nature of Brownian motion, the diffusional particle motion is also random and omnidirectional. Diffusional motion increases with decreasing particle size, whereas it is typically negligible for particles >100 nm.
|
(1) Particle diffusivity
(2) Cunningham slip correction factor
(3) Root mean square displacement |
|
The particle diffusivity D in equation (1) describes a particle’s ability to be moved by the collision with gas molecules. It increases with increasing temperature T and decreasing particle size dp.k is the Boltzmann constant,
|
Execution |
|
These equations are purely deterministic and can easily be solved using a pocket calculator or spreadsheet software.
|
Used in |
|
|
| Coagulation Diffusion |
Read more |
Read also |
|
|
W. Hinds (1999), Aerosol Technology – Properties, Behavior, and Measurement of Airborne Particles, John Wiley & Sons.
A. Einstein (1905), Über die von der molekularkinetischen Theorie der Wärme geforderte Bewegung von in ruhenden Flüssigkeiten suspendierten Teilchen, Ann. Phys. 17: 549-560.
|
Contact

Christof Asbach
Institut für Energie- und Umwelttechnik (IUTA)
Email: asbach@iuta.de