Electrophoretic Light Scattering - ELS
Electrophoretic Light Scattering (ELS) is a technique used to measure the electrophoretic mobility of particles in dispersion, or molecules in solution. This mobility is often converted to zeta potential to enable comparison of materials under different experimental conditions.
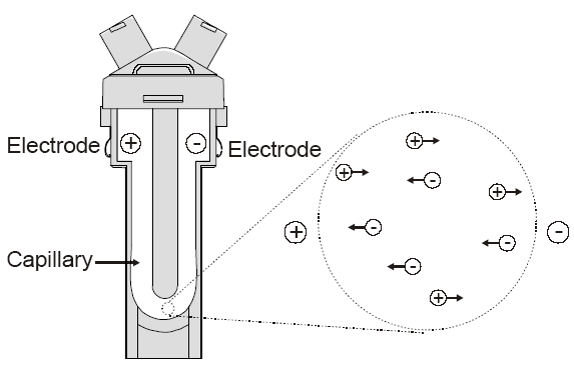
The fundamental physical principle is that of electrophoresis. A dispersion is introduced into a cell containing two electrodes. An electrical field is applied to the electrodes, and particles or molecules that have a net charge, or more strictly a net zeta potential, will migrate towards the oppositely charged electrode with a velocity, known as the mobility, that is related to their zeta potential.
 |

High +ve/-ve Zeta Potential • High interparticle repulsion • Stable suspension |

Low or Zero Zeta Potential • Flocculation, aggregation, agglomeration, Ostwald ripening (small particles dissolving and recrystallizing onto larger ones) • Unstable suspension |
|
| Disposable cuvettes are used for the measurement of zeta potential with the Malvern Panalytical Zetasizer Nano ZSP, ZS, Z and ZS90. | Nanoparticle dispersions may exhibit high or zero net zeta potential affecting the sample stability. |
|
The Malvern Panalytical Zetasizer Nano provides a simple, fast and accurate way to measure zeta potential, and uses a unique disposable capillary cell to ensure that there is no cross contamination between samples
|
Read more |
Read also |
|
https://www.malvern.com/en/products/technology/electrophoretic-light-scattering |
ISO 13099-2 (2012) Colloidal systems - Methods for zeta-potential determination - Part 2: Optical methods.
ASTM E2865 (2012) Standard Guide for Measurement of Electrophoretic Mobility and Zeta Potential of Nanosized Biological Materials. ASTM International, West Conshohocken, PA. www.astm.org
|
Contact

Phil Vincent
Malvern Panalytical
Email: Phil.vincent@malvern.com
